医学essay代写 医学论文作业代写 essay作业代写 医学essay代做
166Name: Course: Topic: The AHRQ Hospital Survey on Patient Safety Culture: A Tool to Plan and Evaluate Patient Safety Programs 医学essay代写 The research that I have selected to present in...
View detailsSearch the whole station
医学研究论文代写 GLP-1 (glucagon-like peptide) is a 30 to 31 amino acid long incretin hormone derived from the tissue-specific post-translation process.
1 Discovery
2 Mechanism of Action
2.1 Brain
2.2 Muscle
2.3 Pancreas
2.4 Liver
2.5 Stomach
3 Role in type 2 diabetes mellitus
4 Previous Research on GLP-1’s Impact on Cardiovascular Protection Along with Diabetes Control
Conclusion
References
Figure 1 Historical Timeline Depicting the Discovery of GLP-1 Analogs
Figure 2 Mechanism of Action of GLP-1 on Target Tissues
Figure 3 Mean Changes from Baseline in Monotherapy and In Combination with GLP-1
GLP-1 (glucagon-like peptide) is a 30 to 31 amino acid long incretin hormone derived from the tissue-specific post-translation process. The level of GLP-1 is lower than normal in type 2 diabetes mellitus; thus, the increased need for GLP-1 analogs in type 2 diabetes patients.
GLP-1 was isolated via protein sequencing and peptide purification and its discovery dates back to the application of recombinant DNA technology in the Stanley Cohen, Paul Berg, and Herb Boyer in early 1970s. The recombinant DNA approaches enabled a quick and efficient prediction of amino acid sequences via nucleotide sequence decoding of cloned recombinant cDNA of mRNAs.
In early 1980s, the laboratory used the approach to interpret proglucagon sequences obtained from genes and cDNAs isolated from anglerfish followed by rat gene sequencing and proglucagon cDNA and later hamster, bovine, and human.
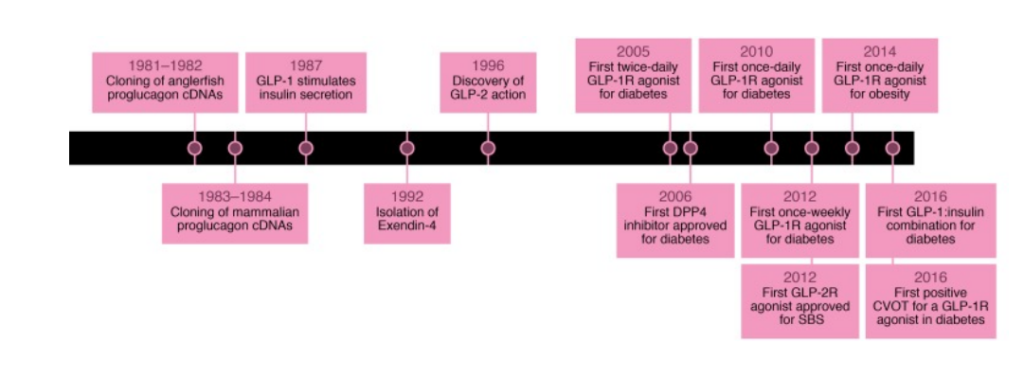
Figure 1 Historical Timeline Depicting the Discovery of GLP-1 Analogs (Drucker et al., 2017)
GLP-1 analogs belong to incretin mimetics drug class that enables the pancreas to produce optimal levels of insulin (hormone that takes glucose/sugar to body tissues) in body parts where it can be used as a source of energy (Dungan and DeSantis, 2019).
These medications limit the rate at which food particles empty the stomach, which enables the body to control after meal (post prandial) blood glucose levels.
By copying the below-mentioned effects of GLP-1 on body parts, GLP-1 analogs control blood glucose levels and appetite through the following mechanism of action:
In brain, hypothalamus is responsible for the control of thirst and appetite. GLP-1 sends a signal to hypothalamus to consume less food and water, which leads to weight loss. However, since GLP-1 dampen the sensation to take in water, there is a risk of dehydration. Thus, special care is required to stay hydrated while on GLP-1 medication (Prasad-Reddy and Isaacs, 2015).
GLP-1 stimulates the synthesis of glucose in the body, a process known as gluconeogenesis. Gluconeogenesis is the conversion of fats and proteins in the body to sugar and glucose for the body to be used as muscle fuel (source of energy). An increase in gluconeogenesis lowers the blood glucose levels by stimulating the uptake of sugar by body cells and increasing the effective use of insulin (Gupta, 2013).
When GLP-1 contacts body glucose, the pancreas is stimulated to release more insulin, thus decreasing the levels of post-meal glycogen in the body (Tran et al., 2017). GLP-1 also lowers the release of glucagon, a hormone that prevents the blood glucose levels from severely falling. Glucagon severely increases the levels of glucose in people who suffer from type 2 diabetes mellitus.
GLP-1 decreases hepatic glucose output that lowers the blood glucose levels. When rate of gluconeogenesis is increased, glucagon receptors are decreased in the liver, which inhibits the formation and stimulation of glucose and its uptake by body cells, thereby decreasing the blood glucose levels.
GLP-1 lowers the release of stomach acid and decrease the process of emptying of food from the stomach, which prolongs the sensation of fullness, thereby limiting the intake of food, ultimately leading to weight loss ((Dungan and DeSantis, 2019).
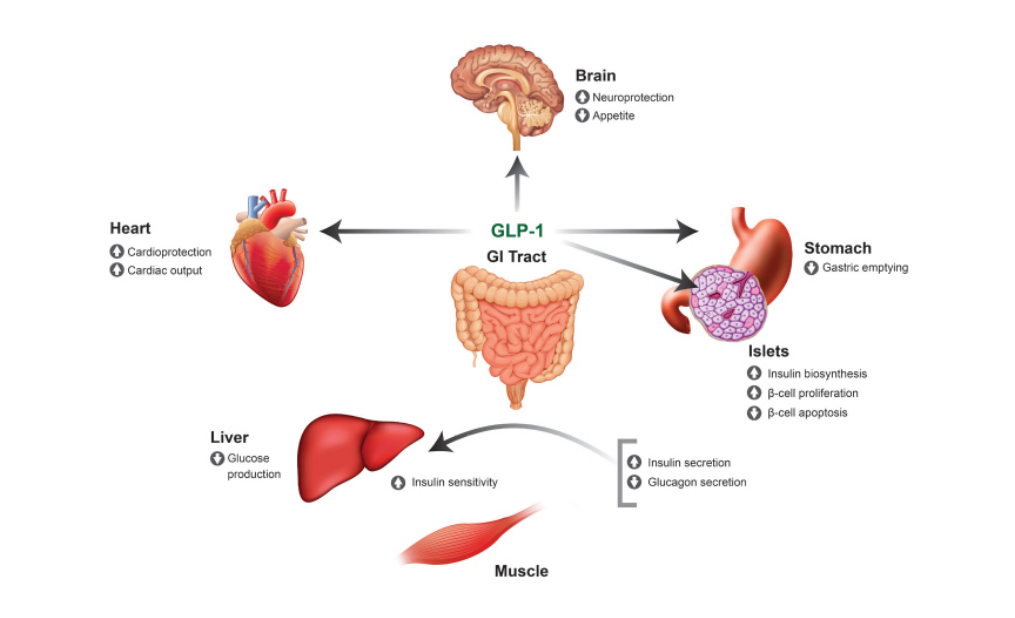
Figure 2 Mechanism of Action of GLP-1 on Target Tissues (Source: ResearchGate)
GLP-1 has numerous benefits in the setting of type 2 diabetes mellitus. Exogenous GLP-1 analogs when intravenously administered to patients with type 2 diabetes mellitus reduce plasma glucose levels to normal fasting concentrations, even in people who previously showed inadequate reaction to oral antihyperglycemic medications (Nauck et al., 1993). The role of GLP-1 analogs observed in type 2 diabetes mellitus patients (Zander et al., 2002) include:
Thus, as per ADA guidelines (Standards of Medical Care in Diabetes, 2016) for glycemic control, GLP-1 analogs are suggested as add-on oral therapy for patients who fail to achieve their target HbA1c levels after 3 months of initial metformin oral therapy (Garber et al., 2016). GLP-1 analogs are also suggested as first-line oral therapy as a metformin oral therapy alternative in patients who suffer metformin-related contraindications.
As per Kayaniyil et al., (2016) meta-analysis, oral therapy with GLP-1 analogs is linked with lowered HbA1c levels from baseline of −0.50% for lixisenatide 20 µg once per day, −0.71% for liraglutide 1.2 mg once per day, −0.42% for exenatide 5 µg BID, −1.03% for liraglutide 1.8 mg once per day, −0.69% for albiglutide 30 mg QW, −1.09% for exenatide 2 mg QW, −0.75% for exenatide 10 µg BID and dulaglutide 1.5 mg QW versus placebo. The figure below shows the mean changes in levels of HbA1c demonstrated in 24 to 52 weeks of clinical studies in the prescribing medication information.
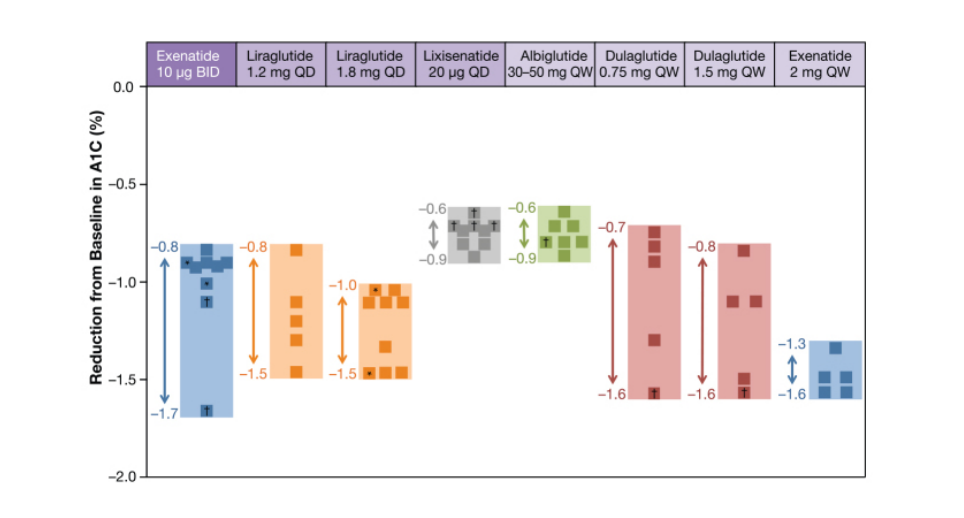
Figure 3 Mean Changes from Baseline in Monotherapy and In Combination with GLP-1 (Source: ADA)
People with type 2 diabetes mellitus are at the risk of cardiovascular complications related to the administration of antihyperglycemic drugs; thus, the cardioprotective effects of antihyperglycemic drugs are of the major interest. As per Food and Drug Administration (FDA) (2008), therapies for type 2 diabetes mellitus should not increase the risk of cardiovascular diseases.
Results indicate that GLP-1 analogs do not aggravate cardiovascular diseases and may show positive potential cardiovascular benefits in type 2 diabetes mellitus. As per the meta-analysis of 25 research projects conducted by Monami et al. (2014), GLP-1 analogs did not lead to adverse CVD effects or major related events such as stroke, cardiovascular death, acute coronary syndromes, nonfatal myocardial infarction and heart failure. Marso et al., (2016) reported similar cardiovascular benefits for semaglutide (GLP-1 analog).
Similarly, GLP-1 has cardiovascular advantages on the vascular endothelium, blood pressure, myocardial ischemia, atherosclerosis progression and inflammation and heart failure (Del Olmo-Garcia and Merino-Torres, 2018). Another study indicates that the effects of GLP-1 analogs on blood pressure, heart rate, inflammation and microvascular function may translate into potential cardiovascular benefits (Heuvelman et al., 2020).
To conclude, GLP-1 analogs are a type of incretin hormone discovered in early 1970s via the efforts of top-notch scientists and research. These analogs have demonstrated positive mechanism of action in pancreas and other muscles leading to lowered glucose levels, reduced HbA1c levels, weight loss and cardio protection in patients with type 2 diabetes mellitus. Not only that, research indicates that GLP-1 analogs can prove to be effective first-line therapy as an alternative to metformin in certain cases.
American Diabetes Association Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S1–S112
Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care 2011;34(Suppl. 2):S264–S271
Del Olmo-Garcia, M. I., & Merino-Torres, J. F. (2018). GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. Journal of diabetes research, 2018, 4020492. https://doi.org/10.1155/2018/4020492
Drucker, D. J., Habener, J. F., & Holst, J. J. (2017). Discovery, characterization, and clinical development of the glucagon-like peptides. The Journal of clinical investigation, 127(12), 4217–4227. https://doi.org/10.1172/JCI97233
Dungan K, DeSantis A. Glucagon-like peptide 1 receptor agonists for the treatment of type 2 diabetes mellitus. UpToDate. May 22, 2019.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016: executive summary. Endocr Pract 2016;22:84–113
Gupta V. Glucagon-like peptide-1 analogues: An overview. Indian J Endocrinol Metab. 2013;17(3):413–421. doi:10.4103/2230-8210.111625
Cardiovascular effects of glucagon-like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovascular research, 116(5), 916–930. https://doi.org/10.1093/cvr/cvz323
Kayaniyil S, Lozano-Ortega G, Bennett HA, et al. . A network meta-analysis comparing exenatide once weekly with other GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus. Diabetes Ther 2016;7:27–43
Marso SP, Bain SC, Consoli A, et al. . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 15 Sep 2016. Electronically published ahead of print (DOI: 10.1056/NEJMoa1607141)
Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014;16:38–47
Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. Published 2015 Jul 9. doi:10.7573/dic.212283
Saraiva FK, Sposito AC. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol 2014;13:142.
Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178–188.
U.S. Food and Drug Administration Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. Silver Spring, Md., U.S. Food and Drug Administration, 2008 [
Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830

更多代写:Cs澳大利亚代考价格 美国托福代考 英国留学生微积分代考 Marketing Management战略营销管理论文代写案例 美国thesis代写 政治学essay作业代写
合作平台:essay代写 论文代写 写手招聘 英国留学生代写
Name: Course: Topic: The AHRQ Hospital Survey on Patient Safety Culture: A Tool to Plan and Evaluate Patient Safety Programs 医学essay代写 The research that I have selected to present in...
View detailsPhysics as a Human Endeavor Never at Rest – A Biography of Isaac Newton 物理学论文课业代写 Isaac Newton is one of the most renowned physicist of the world, having coined concept after conce...
View detailsThe American Revolution 美国历史代写 One of the most important events in the American History is the Revolutionary War, commonly referred to as the American Revolution. In the 1760s, One o...
View detailsMother (1920) by Vsevolod Pudovkin – Movie Review Submitted By [Name of the writer] [Name of the institution] Mother (1920) by Vsevolod Pudovkin – Movie Review 电影评论课业代写 The...
View details