经济考试代写 Econ代写 Midterm代写 problems代写
722Econ391 Sample problems from Midterm 2 经济考试代写 1. Carbon atoms require ________________ bonds to be stable. a. one b. two c. three d. four 2. The core process of refining is 1. 经...
View detailsSearch the whole station
化学代写 A 20.0 mL sample of hypochlorous acid (HClO, Ka = 3.0 × 10−8) of an unknown concentration is titrated with 0.100 M of NaOH(aq).
A 20.0 mL sample of hypochlorous acid (HClO, Ka = 3.0 × 10−8) of an unknown concentration is titrated with 0.100 M of NaOH(aq). What is concentration of the hypochlorous acid if 11.5 mL of NaOH(aq) is needed to reach the equivalence point?
(a) 1.15 × 10−3 M
(b) 3.65 × 10−2 M
(c) 5.75 × 10−2 M
(d) 0.134 M
What is the pH at the equivalence point in the titration from previous problem?
(a) 4.48
(b) 7.00
(c) 9.52
(d) 9.62
(e) 10.08
A 25.00 mL sample of 0.100 M CH3COOH solution (Ka = 1.8 × 10−5) is titrated with 0.100 M NaOH solution. What is the pH of the solution at the points where 24.5 and 25.5 mL of NaOH have been added?
(a) 6.44, 11.00
(b) 6.13, 11.00
(c) 6.44, 9.85
(d) 6.13, 9.85
(e) 7.00, 8.00
If 15.0 mL of a 8.6 × 10−2 M aqueous solution of Ba(OH)2 is titrated with a 0.100 M HCl(aq) solution what is the pH after 5.00 mL of the HCl solution is added?
(a) 8.79
(b) 9.64
(c) 11.32
(d) 12.60
(e) 13.02
Determine the molar solubility of PbCl2 (Ksp = 1.7 × 10–5)
(a) 0.068 M
(b) 4.1 × 10–3 M
(c) 2.9 × 10–3 M
(d) 0.016 M
(e) 0.026 M
In which of the following would PbI2 have the lowest solubility?
(a) Pure H2O
(b) A 0.5 M HI solution
(c) A 0.5 M NaOH solution
(d) A 1.0 M HNO3 solution
(e) A 0.8 M KI solution
What is the concentration of free Ni2+, if 1.83 g of Ni(NO3)2 is dissolved in 1.00 L of 3.0 M NH3 solution? The formation constant for the complex ion [Ni(NH3)6]2+ is Kf = 1.2 × 109 .
(a) 2.1 × 10–12
(b) 1.1 × 10–10
(c) 6.1 × 10–14
(d) 2.8 × 10–12
(e) 1.1 × 10–14
A block of ice containing 1.00 mol of H2O (melting point = 0 °C, ΔHfusion = 6.01 × 103 J/mol) is placed in a dish at 37 °C and allowed to melt. At the point where the ice has just finished melting what is the change in the entropy of the universe? Is this a reversible or irreversible process?
(a) +22.0 J/K, irreversible
(b) −22.0 J/K, reversible
(c) 2.6 J/K, irreversible
(d) 2.6 J/K, reversible
(e) −2.6 J/K, reversible
Consider the reaction shown below:
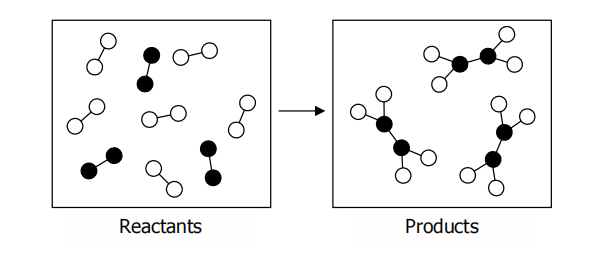
What sign would you expect for the enthalpy, entropy and Gibbs free energy? Assume all bonds are equal in strength and all molecules are gas phase molecules?
(a) ΔS < 0 (negative), ΔH < 0 (negative), ΔG < 0 (negative)
(b) ΔS > 0 (positive), ΔH < 0 (negative), ΔG < 0 (negative)
(c) ΔS > 0 (positive), ΔH > 0 (positive), ΔG > 0 (positive)
(d) ΔS < 0 (negative), ΔH > 0 (positive), ΔG > 0 (positive)
(e) ΔS < 0 (negative), ΔH < 0 (negative), but the sign of DG cannot be determined without more information
Given the following thermodynamic quantities:
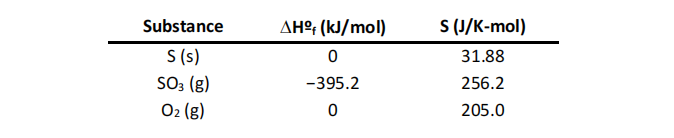
What is the value of DGº at 373 K for the oxidation of solid elemental sulfur .to gaseous sulfur trioxide?
2 S(s) + 3 O2(g) → 2S O3(g)
(a) +740.8 kJ/mol
(b) −61.3 kJ/mol
(c) −740.8 kJ/mol
(d) −728.3 kJ/mol
(e) +61.3 kJ/mol
Given the fact that Ksp for CaSO4 is 2.4 × 10−5, what is ΔG° for the following precipitation reaction
Ca2+(aq) + SO4 2−(aq) → CaSO4(s)
(a) 26.4 kJ
(b) 2.21 kJ
(c) 0 kJ
(d) −2.21 kJ
(e) −26.4 kJ
Balance the following reaction in acidic conditions and determine which side of the balanced equation H+ is located on, and its coefficient.
Cr2O7 2− (aq) + I− (aq) → Cr3+(aq) + IO3− (aq)
(a) Reactant side, coefficient = 14
(b) Reactant side, coefficient = 8
(c) Reactant side, coefficient = 2
(d) Product side, coefficient = 6
(e) Product side, coefficient = 4
Balance the following reaction in basic conditions and determine which side of the balanced equation H2O is located on, and its coefficient.
H2O2(aq) + CIO2(aq) → CIO2−(aq) + O2(g)
(a) Reactant side, coefficient = 2
(b) Reactant side, coefficient = 4
(c) Water does not show up in the balanced equation
(d) Product side, coefficient = 1
(e) Product side, coefficient = 2
If you were asked to construct a voltaic cell using one the following half reactions in the cathode compartment and another in the anode compartment, which combination would give the largest cell voltage (emf)?
Cu2+ (aq) + 2e– → Cu (s) E° = +0.34 V
Zn2+ (aq) + 2e– → Zn (s) E° = –0.76 V
Ni2+ (aq) + 2e– → Ni (s) E° = –0.28 V
a. Cathode = Zn/Zn2+; Anode = Cu/Cu2+
b. Cathode = Cu/Cu2+ ; Anode = Zn/Zn2+
c. Cathode = Zn/Zn2+; Anode = Ni/Ni2+
d. Cathode = Ni/Ni2+; Anode = Zn/Zn2+
e. Cathode = Cu/Cu2+; Anode = Ni/Ni2+
Consider an electrochemical cell based on the following reaction:
2H+ (aq) + Sn (s) → Sn2+ (aq) + H2 (g)
Which of the following actions would increase the measured cell potential?
a. Increasing the size of the tin electrode
b. Adding HNO3(aq) to the anode compartment
c. Adding HNO3(aq) to the cathode compartment
d. Adding NaOH(aq) to the cathode compartment
e. Both (b) and (c) would increase the measured cell potential
The standard cell potential (E°cell) for the reaction below is +0.63 V.
Pb2+(aq) + Zn (s) → Zn2+ (aq) + Pb (s)
What is the cell potential when [Zn2+] = 1.0 M and [Pb2+] = 2.0 × 10−4 M?
a. +0.52 V
b. +0.85 V
c. +0.41 V
d. +0.74 V
e. +0.63 V
The voltaic cell shown below relies on the half reactions
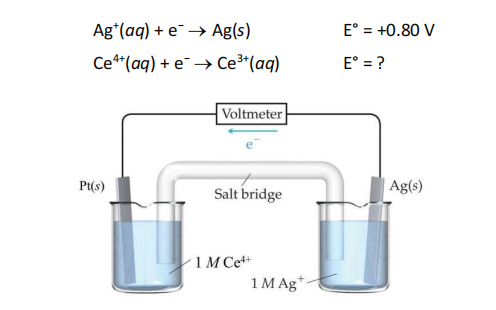
If the measured cell voltage is 0.85 V what is the standard reduction potential for the cathode half reaction Ce4+(aq) + e− → Ce3+(aq)?
a. −0.05 V
b. +0.05 V
c. 1.65 V
d. −1.65 V
e. +0.85 V
If the voltaic cell shown below puts out a voltage of 0.80 V when [Zn2+] = 1.00 M, the pressure of H2(g) is 1.00 atm and the temperature is 298 K, what is the pH in the cathode compartment?
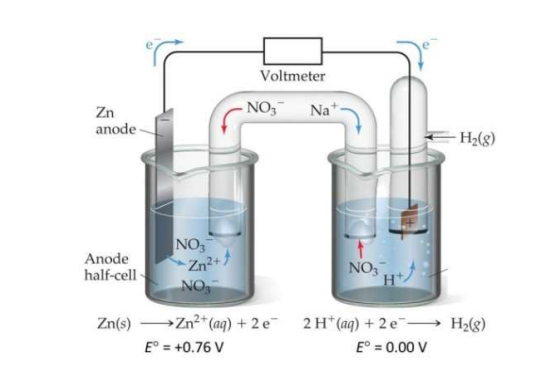
(a) pH = 0.21
(b) pH = 0.34
(c) pH = 0.68
(d) pH = 1.35
(e) pH = −0.68

更多代写:Java代写 澳大利亚宏观经济代考 英国thesis代寫 summary essay Speeches代写 算法作业代写
合作平台:essay代写 论文代写 写手招聘 英国留学生代写
Econ391 Sample problems from Midterm 2 经济考试代写 1. Carbon atoms require ________________ bonds to be stable. a. one b. two c. three d. four 2. The core process of refining is 1. 经...
View detailsECON 338 Midterm 经济期中代写 1.Answer one and only one of the following two questions: a. Is a description of the actual objects of our desires relevant to praxeology, 1.Answer one an...
View detailsCOEN 174 Software Engineering Midterm1 (50 pts) 软件工程代写 For each of the systems described in a) and b), select the most appropriate software process model from the list...
View details